Tuesday, 26 July 2016
Sunday, 17 July 2016
MFC - first weekend

First tests of bioreactor powered on horse meat. With the courtesy of House Layer in Kranj, we started using a nice place for one-month testing. Before coming to Kranj preparations were mostly getting all materials and equipment needed for tests. Methylan blue, different measuring pots (Mikro+Polo), Lactobacillus culture (Biotechnical centre Naklo), Agar, agar (food store), Inox rods for electrodes (MDM), carbon (Samson), digital multimeter (IC Elektronik), different plastic containers ... (hardware shop).


Salt bridge initial formula 300 ml of water, 30 ml of salt (NaCl) and 5 g of Agar agar. Later ratio was 10% of salt and 5% of agar agar.
Minced meat was processed in a blender to smaller pieces. Meat 17% (1/2 kg and 2 1/2 of water)
Lactobacillus - bulgaricus and Streptococcus thermophilus
Toner was added to epoxy glue
Preparation of bioreactor was mostly handled by Sara Zupanc and Benjamin Drakslar who were also suggested making additional tests on the different percentage of content of the cultures.
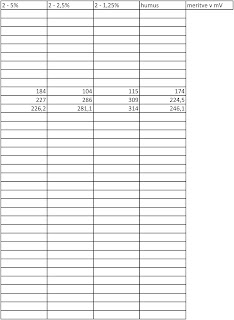
And the results after the end of the weekend.
Saturday, 9 July 2016
micro fuel cell - preparation
We had a wonderful meeting with Sara and Benjamin in the third biggest brewery in Slovenia - Kranjska pivnica. A short list of ingredients and possible links is added below. Tests will start soon.
mediators:
microbiological cultures
Lactobacillus:
biotehnični center naklo http://www.bc-naklo.si/
cheesemaking
agar agar
containers
Tuesday, 12 April 2016
First dissolving experiments
The first experiments with dissolving horse meat, using different acids and catalysts.
Catalysts used:
The test setup also included a peltier element SP1848 attached to the bottom of the test vessel. The element sat on a heat sink that was cooled with a fan. Produced voltage was measured with a multi-meter. The heat conduction path between peltier element and test vessel was reasonably good, but not perfected with silicon paste of such.
Catalyst: K₂Cr₂O₇ solution, unknown amount, maybe 10 ml
Catalyst: K₂Cr₂O₇ solution, 10 ml
The different between this and the first experiment caused great concern. It was theorized that the acid solution was too old and had somehow lost its potency.
An observation unrelated to the experiment was made. The soda used to neutralize the acid afterwards caused the solution to cool down to 8 °C.
Catalyst: K₂Cr₂O₇ solution, 10 ml
Catalyst: Ag₂SO₄ solution, 10 ml
Acid: H₂SO₄, 50 ml
No great heat production was observed after adding acid to catalyst. Adding meat caused increase to temperature, with 42 °C observed at highest. Voltage over Peltier element was 42 mV. The meat was visibly dissolving and becoming black on the edges. The experiment was terminated after about 10 minutes, with a large portion of meat still remaining. The color of solution was whitish pink.
Catalyst: K₂Cr₂O₇ solution, 10 ml
Acid: H₂SO₄, 50 ml
This was a repeat experiment of experiment 1, in order to replicate the heat production on adding acid, and to measure heat produced by dissolving meat alone.
Experimental setup
Catalysts used:
- Potassium dichromate K₂Cr₂O₇. Solution 1 gram mixed to 50 ml tap water.
- Silver sulphate Ag₂SO₄. Solution 1 gram in 50 ml acid
- Sulphuric acid H₂SO₄, 1.8 g/ml
- Hydrochloric acid HCl in water solution, 37.5 %
- Hydrochloric acid HCl in alcohol solution, 1.25 M
The test setup also included a peltier element SP1848 attached to the bottom of the test vessel. The element sat on a heat sink that was cooled with a fan. Produced voltage was measured with a multi-meter. The heat conduction path between peltier element and test vessel was reasonably good, but not perfected with silicon paste of such.
Experiment 1
Catalyst: K₂Cr₂O₇ solution, unknown amount, maybe 10 ml
Acid: H₂SO₄, 50 ml
Strong heating to 72 °C was observed after adding acid to catalyst. Adding meat increased to heat slightly to 79 °C, with voltage over Peltier element reaching 250 mV at highest. The meat dissolved completely in very short period of this, perhaps one minute. The color of resulting sludge was very dark red, almost black.
Afterwards, it was considered a mistake to add the meat immediately after acid, as the effect of meat heat was shadowed by heating caused by catalyst-acid reaction.
Experiment 2
Catalyst: K₂Cr₂O₇ solution, 10 ml
Acid: 37.5 % HCl, 50 ml
Compared to previous experiment, very little heating was observed after adding acid to catalyst - only 29 °C was observed. The meat did not seem to dissolve at all, not did it produce heat. The experiment was terminated after waiting for maybe two minutes.
An observation unrelated to the experiment was made. The soda used to neutralize the acid afterwards caused the solution to cool down to 8 °C.
Experiment 3
Catalyst: K₂Cr₂O₇ solution, 10 ml
Acid: HCl 1.25 M, 50 ml
This experiment went similarly to experiment 2. It was (weakly) concluded that hydrochloric acid is not suitable for this project.
After the experiment, some Ag₂SO₄ was added to the solution, just to quickly test if this catalyst could improve the matters. No heat production was observed.
This experiment went similarly to experiment 2. It was (weakly) concluded that hydrochloric acid is not suitable for this project.
After the experiment, some Ag₂SO₄ was added to the solution, just to quickly test if this catalyst could improve the matters. No heat production was observed.
Experiment 4
Catalyst: Ag₂SO₄ solution, 10 ml
No great heat production was observed after adding acid to catalyst. Adding meat caused increase to temperature, with 42 °C observed at highest. Voltage over Peltier element was 42 mV. The meat was visibly dissolving and becoming black on the edges. The experiment was terminated after about 10 minutes, with a large portion of meat still remaining. The color of solution was whitish pink.
This experiment was considered relatively successful since some heat was produced and the reaction did not proceed too fast.
Experiment 5
Catalyst: K₂Cr₂O₇ solution, 10 ml
This was a repeat experiment of experiment 1, in order to replicate the heat production on adding acid, and to measure heat produced by dissolving meat alone.
Adding acid to catalyst caused temperatures above 80 °C. The solution was left to cool until its temperature was at 25 °C before meat was added.
Adding meat resulted in temperature rising to 31°C, with voltage over Peltier element at 34 mV. This went on for maybe 5 minutes, after which temperature dropped somewhat, even though the piece of meat was not completely dissolved.
Note that this experiment was conducted after sunset, when outside air coming in from the window was substantially colder than during the other experiments - maybe 5 °C difference. Also note that the meat was in one 5 gram piece this time.
Conclusions
In light of different between experiment 1 and experiment 5, probably all the combinations did work; the great amount of heat in the first reaction just set unrealistic expectations.
The method used here does not produce very useful amounts of power. The amount of heat generated was below expectations.
It could be useful to have instruments for voltage and temperature that are connected to computer taking automated longer time series.
The method used here does not produce very useful amounts of power. The amount of heat generated was below expectations.
It could be useful to have instruments for voltage and temperature that are connected to computer taking automated longer time series.
Wednesday, 23 March 2016
KPK protocol (in Slovene)
KEMIJSKA POTREBA PO KISIKU (KPK)
Sorry Otto - I'll try to find out English version of a project - protocol is here for measurements of all substances (ingredients)
Test kemijske potrebe po kisiku (KPK) nam pove, kakšna količina kisika je potrebna za kemijsko oksidacijo organskih snovi v vzorcu z močnim kemijskim oksidantom. KPK je torej kisikov ekvivalent vsebnosti organske snovi v vzorcu in je indirektno merilo za količino organske snovi. Kot moča n kemijski oksida nt upora bimo ka lijev dikroma t (K2Cr2O7), ki večino orga nskih snovi oksidira do 95 % – 100 % teoretske vrednosti. K popolnejši oksida ciji orga nskih spojin pripomorejo še koncentrira na H2SO4, visoka tempera tura (150 °C , 2h) in ka ta liza tor, Ag2SO4.
Test kemijske potrebe po kisiku (KPK) nam pove, kakšna količina kisika je potrebna za kemijsko oksidacijo organskih snovi v vzorcu z močnim kemijskim oksidantom. KPK je torej kisikov ekvivalent vsebnosti organske snovi v vzorcu in je indirektno merilo za količino organske snovi. Kot moč
Za vzorce z znano kemijsko sestavo lahko vrednost KPK izračunamo. Na primer za 1 g glukoze je izračun naslednji:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
Za oksidacijo 1 mola glukoze (= 180 g ) potrebujemo 6 molov kisika (192 g ). KPK vrednost za 1 g glukoze je potem 1,07 g O2.
Za kompleksnejše vzorce pa je potrebno izvesti meritev. Kot oksidant se uporablja kalijev dikromat, ki učinkovito oksidira večino organskih spojin (95-100% teoretične vrednosti). Za test KPK vzorčimo v steklene posode in jih analiziramo takoj, sicer jih konzerviramo (s koncentrirano žveplovo kislino, da pade pH na približno 2). Če so prisotne suspendirane snovi, vzorec pred testom homogeniziramo. Vzorce z veliko vrednostjo KPK ustrezno redčimo.
Za test KPK vzorec v prebitku standardne raztopine kalijevega dikromata segrevamo 2 uri pri 150 °C v močno kislem mediju (zakisano z žvepleno kislino). Kot katalizator uporabimo srebrov sulfat, ki pospeši oksidacijo alifatskih spojin. Pri tem poteče oksidacija in del kalijevega dikromata se reducira:
Cr2O72+ → Cr3+
Pri odprti refluksni metodi, ki se uporablja za večje vzorce in je standardni KPK test, se preostanek kalijevega dikromata ugotovi titrimetrično z amon-železovim (II) sulfatom. Količina reduciranega kromata je sorazmerna s porabo kisika in jo izrazimo v obliki ekvivalenta kisika. Slaba stran te metode je predvsem v veliki porabi kemikalij.
Pri zaprti refluksni metodi (slika 2) pa se preostali kalijev dikromat ugotovi kolorimetrično. Cr3+ namreč močno absorbira svetlobo pri 600 nm, medtem ko dikromat skoraj nič pri 600 in zelo močno pri 420 nm. Nastalo zeleno barvo (Cr3+) odčitamo spektrofotometrično pri 600 nm.
Slika 2: Kolorimetrična zaprta refluksna metoda za določanje KPK. Večja kot je vrednost KPK v testiranem vzorcu, bolj intenzivna zelena barva se pojavi na račun redukcije Cr2O72+ v Cr3+.
Za izvedbo KPK testa pripravimo (reagenti so že pripravljeni!):
R1: V 500 mL destilira ne vode ra ztopimo 10,216 g K2Cr2O7 (sušenega 2h na 105 °C ). Doda mo 167 mL koncentrira ne H2SO4 in dopolnimo z destila tom do 1000 mL.
R2: 10 g Ag2SO4 ra ztopimo v 1000 mL koncentrira ne H2SO4.
Rea gent za KPK: R1 in R2 previdno in poča si zmeša mo v ra zmerju 1,5:3,5 (150 mL R1 + 350 mL R2). To storimo en da n pred upora bo, ker se rea gent močno segreje.
R1 za slepi vzorec: v 1,67 mL koncentrira ne H2SO4 doda mo destila t do 10 mL.
Slepi vzorec za KPK
Zmeša mo 2,5 mL destila ta , 1,5 mL R1 za slepi vzorec in 3,5 mL R2. Proti slepemu vzorcu merimo a bsorba nce vseh osta lih vzorcev in umeritvenih ra ztopin na spektrofotometru.
Umeritvena krivulja
Pripra vimo 0, 100, 300, 500, 700 in 1000 mg/L koncentra cije glukoze v destila tu.
2,5 (1,25)mL posa meznih ra ztopin doda mo k 5 (2,5)mL rea genta za KPK in 2 (1,5) h izva ja mo oksida cijo pri 150 °C . Na vajah uporabljamo vrednosti v oklepajih!!!
Preglednica 2: Izmerjene absorbance (pri 600 nm) za standardne raztopine glukoze
koncentracija glukoze
|
KPK – vrednost (izr
|
absorbanca pri 600 nm
|
0 mg/L
|
||
100 mg/L
|
||
300 mg/L
|
||
500 mg/L
|
||
700 mg/L
|
||
1000 mg/L
|
1070 mg
|
Na spektrofotometru a bsorba nca m umeritvenih krivulj pripišemo ka r ustrezne KPK vrednosti.
Izvedba KPK testa
Pripravimo si 2 homogenizirani raztopini tropin. 90 mg tropin zmiksamo z ultraturaxom v 90 ml destilirane vode. Eno od raztopin kuhamo 2 uri (termična predobdelava). Z enakimi raztopinami tropin bomo delali v testu BMP.
Vzorce predhodno razredčimo (1:100 in 1:200) in redšitve upoštevamo pri končnem izračunu vrednosti KPK.
Test izvedemo v dveh paralelnih ponovitvah. V epruvete HACH z navojnim zamaškom in teflonskim tesnilom odpipetira mo po 5 (2,5) mL rea genta za KPK. Doda mo 2,5 (1,25) mL redčenega vzorca in vsebino premeša mo z meša lnikom. Vsa ko epruveto odpremo, za premo in posta vimo za 2 (1,5)h na 150 °C . Ohla jene epruvete centrifugira mo 10 min pri 3000 rpm, da odstra nimo na sta lo oborino.
Ka lijev dikroma t se pri redukciji oba rva zeleno. Intenziteta ba rve je v sora zmerju s kisikom, ki se je pora bil za oksida cijo orga nske snovi v vzorcu.
Ob merjenju a bsorba nce vzorcev na m spektrofotometer na podla gi predhodno izmerjene umeritvene krivulje izra čuna KPK vrednosti. Pri končnem rezulta tu je potrebno upošteva ti še fa ktor redčitve.
Preglednica 3: Izmerjene in prera čuna ne vrednosti KPK
vzorec
|
redčitev
|
odčit
|
končni KPK (mg/L)
|
povprečna vrednost KPK (mg/L)
|
|
short English version (also on: https://en.wikipedia.org/wiki/Chemical_oxygen_demand)
The Dichromate Chemical Oxygen Demand (COD) test measures the oxygen equivalent of the
amount of organic matter oxidizable by potassium dichromate in a 50% sulfuric acid solution.
Generally, a silver compound is added as a catalyst to promote the oxidation of certain classes of
organics, and a mercuric compound may be added to reduce interference from the oxidation of
chloride ions by the dichromate. End products are carbon dioxide, water, and various states of the
chromium ion.
Chemical reactions
In the oxidation of organic materials by dichromate in sulfuric acid, most of the carbon is converted
to carbon dioxide while any hydrogen present in the organic compound is converted to water.
Other elements also may be oxidized.
Chemical oxygen demand results are usually expressed by the amount of oxygen consumed
during the oxidation of organic matter. When oxygen is used as the primary oxidant in the
oxidation of potassium acid phthalate, the equation below describes the reaction.
Seven and one-half molecules of oxygen (O2) consume one molecule of potassium acid phthalate
(KHP). On a weight basis, the theoretical oxygen demand for KHP is 1.175 mg O2 per mg KHP.
There are two basic methods, titrimetric and colorimetric, for determining the amount of chromium
in a particular valence state.
The Dichromate Chemical Oxygen Demand (COD) test measures the oxygen equivalent of the
amount of organic matter oxidizable by potassium dichromate in a 50% sulfuric acid solution.
Generally, a silver compound is added as a catalyst to promote the oxidation of certain classes of
organics, and a mercuric compound may be added to reduce interference from the oxidation of
chloride ions by the dichromate. End products are carbon dioxide, water, and various states of the
chromium ion.
Chemical reactions
In the oxidation of organic materials by dichromate in sulfuric acid, most of the carbon is converted
to carbon dioxide while any hydrogen present in the organic compound is converted to water.
Other elements also may be oxidized.
Chemical oxygen demand results are usually expressed by the amount of oxygen consumed
during the oxidation of organic matter. When oxygen is used as the primary oxidant in the
oxidation of potassium acid phthalate, the equation below describes the reaction.
Seven and one-half molecules of oxygen (O2) consume one molecule of potassium acid phthalate
(KHP). On a weight basis, the theoretical oxygen demand for KHP is 1.175 mg O2 per mg KHP.
There are two basic methods, titrimetric and colorimetric, for determining the amount of chromium
in a particular valence state.
Tuesday, 22 March 2016
22 March meeting
Hostel Celica kafić: Kristijan, Sara, Benjamin and Z
The first experiment will be chemical. Horse meat will decompose in sulfuric acid H2SO4.
What we need
Horse meat
Sulfuric acid, H2SO4
Potassium dichromate, K2Cr2O7
Glass Petri dish
Peltier device
Joule thief
Heat sink
additional
ph meter
V / A meter
thermometer
latex gloves
safety goggles
optional:
Heat resistance and thermal insulation
for replay
The first experiment will be chemical. Horse meat will decompose in sulfuric acid H2SO4.
What we need
Horse meat
Sulfuric acid, H2SO4
Potassium dichromate, K2Cr2O7
Glass Petri dish
Peltier device
Joule thief
Heat sink
additional
ph meter
V / A meter
thermometer
latex gloves
safety goggles
optional:
Heat resistance and thermal insulation
For the second test, the plan is to work with microbial fuel cells but needs some time for preparation
for replay
Monday, 21 March 2016
Domestication of a horse
Maybe just another insight in the horse theme - in this video blog theme horse there are some answers on why some animals as horse are domesticable and others as zebra aren't.
Subscribe to:
Posts (Atom)